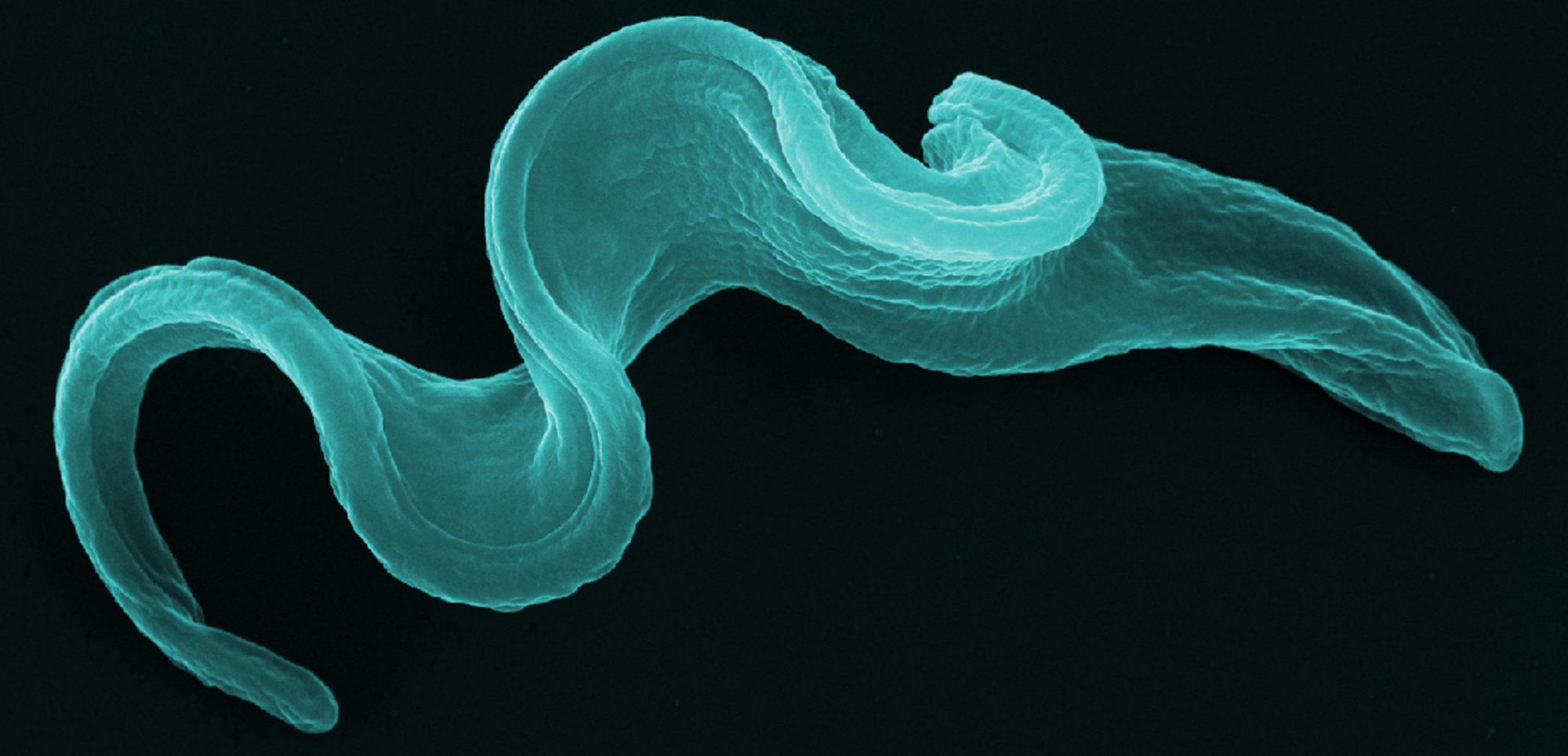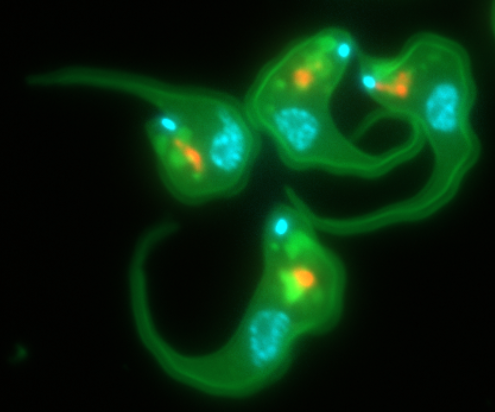
Trypanosoma brucei labelled for DNA (blue), cholesterol uptake (green) and Golgi compartment (orange) 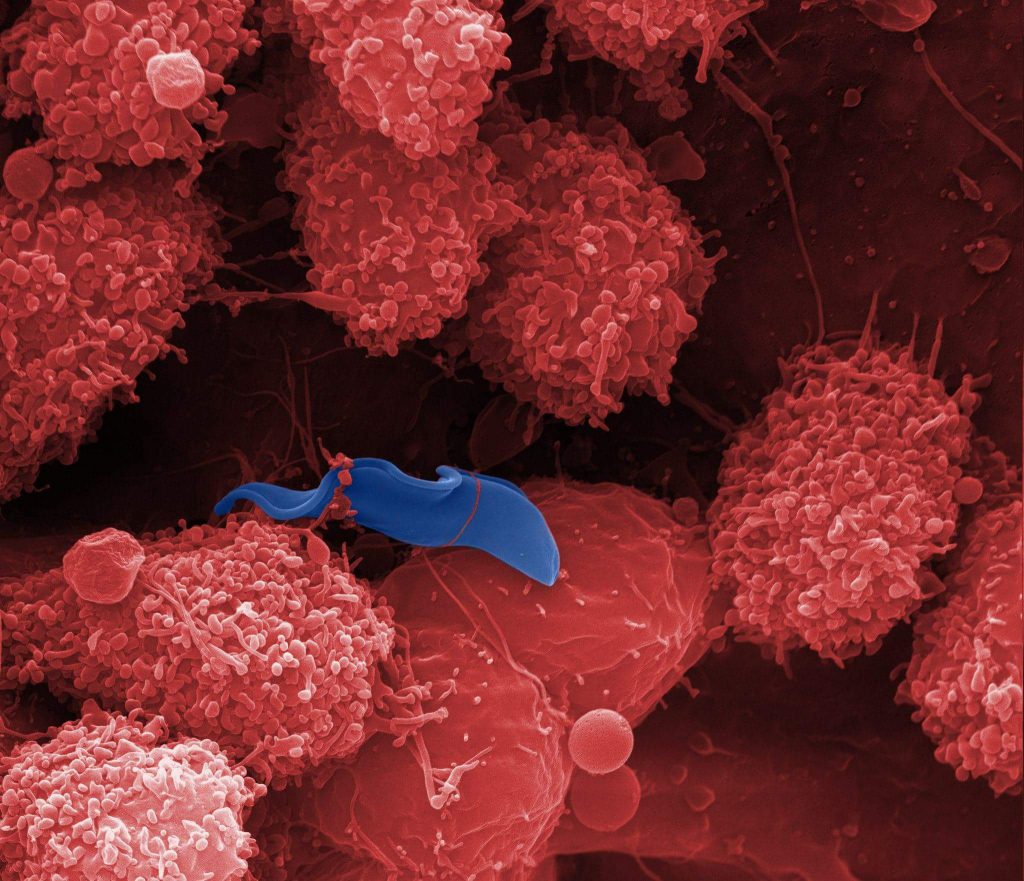
False-coloured SEM image. Trypanosoma brucei and lymphoid cells in the mouse liver 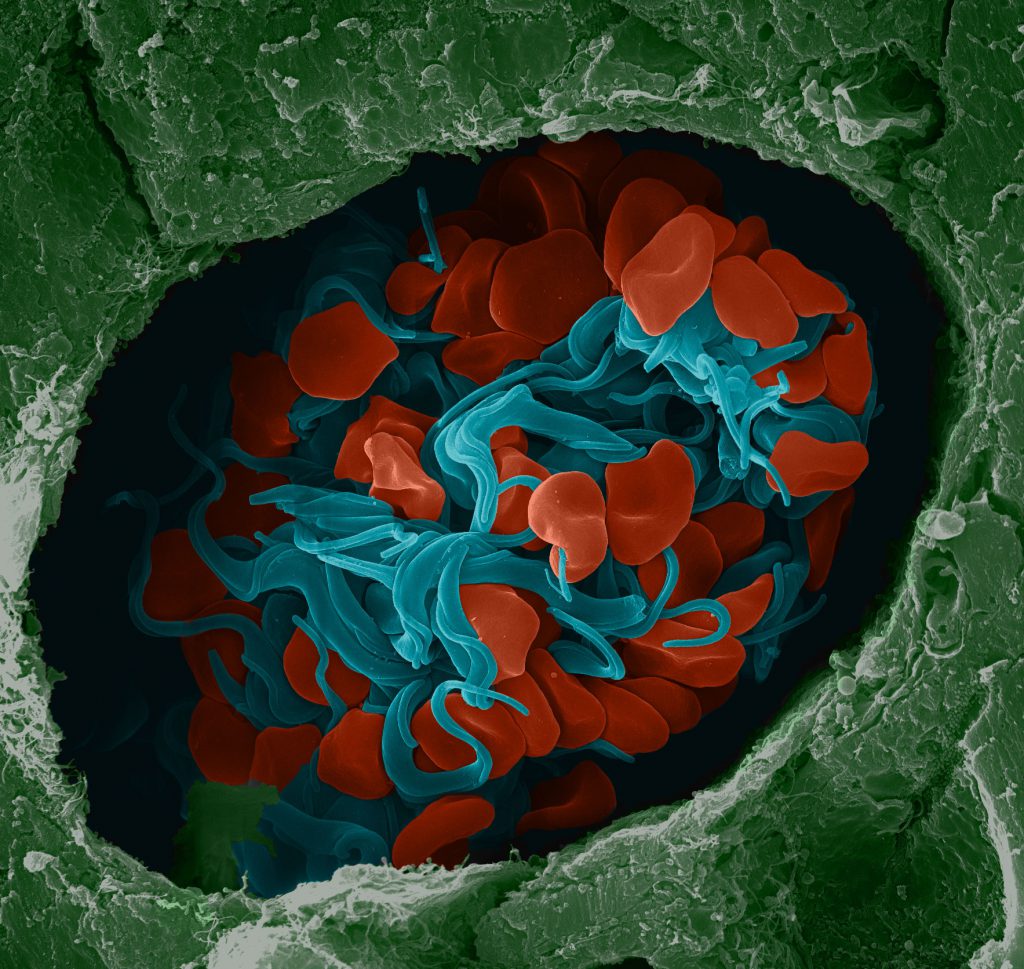
False-coloured SEM image of trypanosomes (blue) and red blood cells (red) in a mouse brain vessel
Thèmes de Recherche
Les trypanosomes Africains sont des Protozoaires parasites véhiculés par la mouche tsé-tsé. Ils provoquent la maladie du sommeil chez l’homme et la nagana chez le bétail. La première maladie tue plusieurs centaines de milliers de personnes par an, et la deuxième entrave considérablement la production de viande et de lait sur plus d’un tiers du continent Africain. Par conséquent, les trypanosomes constituent un fléau majeur, et l’élaboration de stratégies de lutte s’impose de façon évidente.
Par ailleurs, ces organismes se sont révélés être un remarquable modèle d’étude, et ce, à deux niveaux. D’abord les caractéristiques de leur développement autorisent l’étude approfondie, c’est-à-dire génétique, des stratégies fascinantes par lesquelles les parasites en général échappent aux défenses de leurs hôtes. Il s’agit en particulier de la variation antigénique (changement continuel de la surface du parasite), de l’immunosuppression (dérèglement actif du système immunitaire de l’hôte), de la résistance à la lyse par un facteur du sérum humain, l’apolipoprotéine L1 (ApoL1), ainsi que d’une restriction spatiale de leurs échanges avec le milieu extérieur (l’ensemble des récepteurs étant localisés dans un renflement de la membrane cytoplasmique moins accessible au système immunitaire). La combinaison de ces stratégies permet au parasite non seulement d’échapper en permanence à la réponse immunitaire, mais aussi d’utiliser cette réponse à son profit.
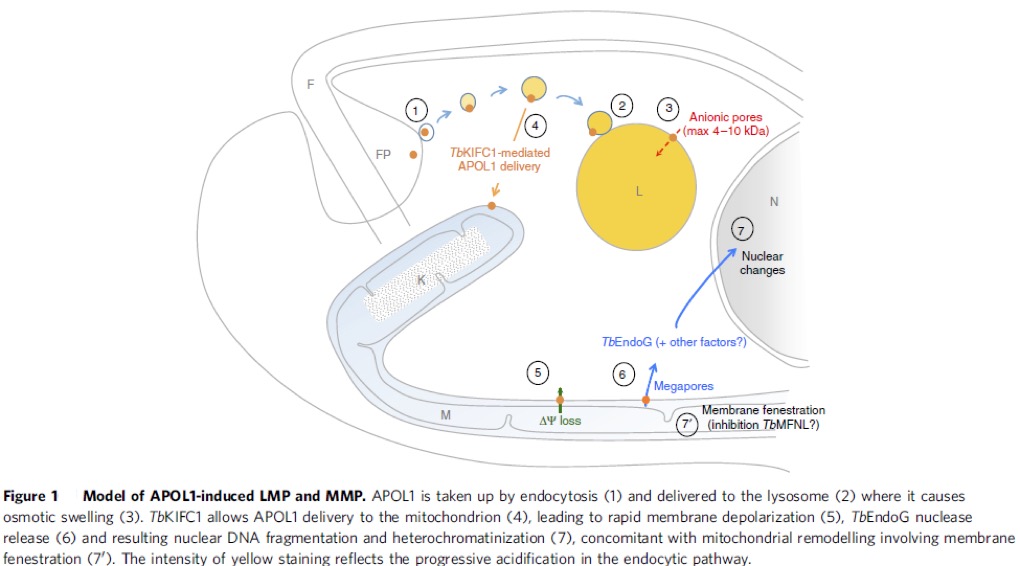
Trypanosomiasis Pathogeny.
African trypanosomes are among the deadliest parasites in Africa for humans and animals. Trypanosoma brucei (T. b.) is the prototype of the species having T. b. brucei causing Nagana and other diseases in animals and T. b. rhodesiense and T. b. gambiense causing sleeping sickness in humans. This disease is currently epidemic in Angola, Democratic Republic of Congo and Sudan, and prevalent in Cameroon, Central African Republic, Chad, Congo, Ivory Coast, Guinea, Mozambique, Uganda and United Republic of Tanzania. Apolipoprotein L1, the only secreted member of a family of 6 proteins (APOL1 to -6), kills very efficiently non-pathogenic trypanosomes, such as T. b. brucei (1). APOL1 is carried by high-density lipoprotein particles (HDL) in normal human serum (NHS). Pathogenic rhodesiense and gambiense trypanosomes can neutralize this APOL1 protein and therefore infect humans. We know how rhodesiense does this: by producing the SRA (serum resistance associated) protein that blocks the lytic effects of APOL1 by direct protein-protein interaction (1, 2). We also know how APOL1 kills: by inserting itself into the lysosomal membrane of the parasite and opening an anionic pore that causes the osmotic swelling of the lysosome and consequently the death of the parasite (3, 4). We recently expanded our knowledge to how this cell death mechanism occurs and found that APOL1 also inserts in the mitochondrion of the parasite, which induces membrane fenestration and TbEndoG endonuclease leakage, which causes nuclear DNA breakage (5, Fig. 1). This DNA damage is the irreversible effect of cell death. We also found howT. b. gambiense blocks the effects of ApoL1. As predicted (6, 7), this mechanism is very different to that found in T. b. rhodesiense and involves three different levels of resistance (8): i) The expression of the protein TgsGP (9) that stiffens endosomal membranes; ii) Reduced sensitivity to APOL1 requiring cysteine protease activity, and iii) Inactivation of the TbHpHbR receptor (10) that limits the uptake of APOL1 into the parasite (11).
Research objectives
Through more than 20 years of intense research we have identified two targets to fight Human African Trypanosomiasis: SRA and TgSGP. These proteins confer resistance to the human APOL1 toxin protein in T. b. rhodesiense and T. b. gambiense; correspondingly. Physical excision of the genes coding for SRA in rhodesiense and TgSGP in gambiense, renders both sous-species sensitive to APOL1, and therefore, non-infective to humans. Both genes are not essential for normal growth and they are species-specific and not existent in humans; adding to their interest as therapy targets. Through structural analyses of SRA and TgSGP we will identify sensitive regions to design drugs useful for therapy. Of all cases of trypanosomiasis, 98% are due to gambiense and current treatment is toxic to patients, therefore, it is urgent to find efficient, non-toxic drugs. By studying the interaction of TgSGP with membranes, we aim to identify the mechanism that leads to exclusion of APOL1 from endosomes. Combining both structural and functional analyses we will identify the target regions in TgSGP for drug design and therapy. The importance of these studies has been highlighted by our development of an engineered therapeutic APOL1 protein that kills non-pathogenic and pathogenic trypanosomes, including T. b. gambiense, in vivo (12). This type of protein engineering will be faster with structural information.
More broadly, understanding such a unique mechanism at molecular level, will impact our knowledge of numerous host-pathogen interactions that engage the endocytic pathway.
References
1- Vanhamme L, et al. (2003). Nature 422: 83-87.
2- Xong HV, et al. (1998). Cell. 95: 839-46
3- Pérez-Morga D, et al. (2005). Science 309: 469-72
4- Pays, E, et al. (2005). Nature Reviews Microbiol. 4: 477-486.
5- Vanwalleghem G, et al. (2015). Nature Comm. 6:8078.
6- Cécile Felu (2006). Ph. D. thesis. Faculté des Sciences.ULB.
7- Felu C, et al. (2007). Am J Trop Med Hyg. 76: 922-9.
8- Uzureau P, et al. (2013). Nature. 501: 430-4.
9- Berberof M, et al. (2001). Mol Biochem Parasitol. 113(1):127-38.
10- Vanhollebeke B, et al. (2008). Science. 320:677-81
11- Pays E, et al. (2014). Nature Rev Microbiol. 12:575-84.
12- Fontaine F, et al. (2017). Nature Microbiol. 2:1500-1506.